Manuscript accepted on : October 24, 2008
Published online on: 04-04-2016
Formulation,Optimization and Evaluation of Ocular Inserts of Ciprofloxacin Hydrochloride
Pankaj Kumar Sharma1, Arun Kumar1, Meenakshi Bajpai1, Monika Sachdeva1 and Pradeep Kumar2
1College of Pharmaceutical Sciences, Raj Kumar Goel Institute of Technology, Ghaziabad India.
2Department of Pharmaceutical Analysis, PES College of Pharmacy, Bangalore India.
Corresponding Author E_mail: pradeep_alpine@yahoo.co.in
ABSTRACT: Ocular inserts of Ciprofloxacin hydrochloride using Gelrite, HPMC, PVP and Carbopol were prepared by surface casting method and evaluated for uniformity of thickness, weight, drug content, surface pH, percent moisture absorption , percent moisture loss and in vitro release study. It can be concluded from the study that gelrite based ocular inserts of Ciprofloxacin hydrochloride shows constant release upto 6 hrs and can be effectively used for sustained topical ocular delivery.
KEYWORDS: Gelrite; HPMC; PVP; Carbopol; Ciprofloxacin hydrochloride; ocular insert
Download this article as:| Copy the following to cite this article: Sharma P. K, Kumar A, Bajpai M, Sachdeva M, Kumar P. Formulation,Optimization and Evaluation of Ocular Inserts of Ciprofloxacin Hydrochloride. Biosci Biotechnol Res Asia 2008;5(2). |
Introduction
The eye as a portal for drug delivery is generally used for local therapy against systemic therapy in order to avoid the risk of eye damage from high blood concentrations of the drug, which is not intended. The unique anatomy, physiology and biochemistry of the eye render this organ impervious to foreign substances, thus presenting a constant challenge to the formulator to circumvent the protective barriers of the eye without causing permanent tissue damage.1, 2
Most ocular treatments like eye drops and suspensions call for the topical administration of ophthalmically active drug to the tissues around the ocular cavity. These dosage forms are easy to instill but suffer from the inherent drawback that the majority of the medication they contain is immediately diluted in the tear film as soon as the eye drop solution is instilled into the cul-de-sac and is rapidly drained away from the precorneal cavity by constant tear flow and lacrimo-nasal drainage.1,2
Therefore only a very small fraction of the instilled dose is absorbed by the target tissue for this reason, concentration solutions and frequent dosing are required for the instillation to achieve an adequate level of therapeutic effect. One of new classes of drug delivery system, polymeric film ocular drug delivery systems, which are gaining worldwide accolade, release drugs at a pre-programmed rate for a longer period by increasing the precorneal residence time.2-5
The poor accessibility of a number of ocular regions to systemic circulation makes local delivery via topical administration the preferred route for the treatment of ocular diseases. Typical conditions that require ocular administration include eye infections and ocular disorders (like conjunctivitis and glaucoma).
The biological barriers involved for ocular delivery are the permeability barriers posed by cornea and other regions, as well as the tear washout and blinking reflexes designed to remove foreign substances from the eye. Furthermore, the ocular region is very sensitive and cannot withstand high local concentrations of drugs or vehicles without irritation. Because of these limitations, designing formulations and delivery systems for topically applied ophthalmic drugs is challenging. It requires thorough understanding of physiological basis of the protective mechanism designed by the eye, which allow only 1-10% of topically applied dose to be absorbed locally.5
Attempts to improve ocular bioavailability have been focussed on overcoming precorneal solution drainage through manipulation of solution viscosity with polymers, use of muco-adhesive polymers, collagen shields, gels, nano-particles, liposomes, latex systems, iontophoresis etc. These ocular drug delivery systems, while limited in providing ideal bioavailability profiles, do provide opportunities for improvement. A better approach of ocular product behaviour coupled with formulation optimization can lead the way to development of newer ocular drug delivery systems .6
Ocular Route and Drug Delivery Systems
Traditional dosage forms for delivery of drugs in the eye have been and remain, solutions and ointments; however, as a consequence of its function as the visual apparatus, Mechanisms are strongly developed for the clearance of foreign materials from the cornea to preserve visual acuity. This presents problems in the development of formulations for ophthalmic therapy. A large proportion of the topically applied is immediately diluted in the tear film and excess fluid spills over the lid margin and the remainder is rapidly drained into the nasolacrymal duct. A proportion of the drug is not available for therapeutic action since its binds to the surrounding extra orbital tissues. These processes lead to a typical corneal contact time of about 1 to 2 minutes in humans, for an instilled solution, and an ocular bioavailability that is commonly less than 10%.4,5
To optimize ocular drug delivery system, the following characteristics are required:
A good corneal penetration
A prolonged contact time with the corneal epithelium
A simplicity of instillation for the patient
A non-irritative and comfortable form the system should not provoke lachrymation and reflex blinking
Appropriate rheological properties
Several new preparations have been developed for ophthalmic use. Successful results were obtained with inserts and diffusion-controlled systems, although these preparations present soon disadvantages, such as noncompliance, specially by elderly people, and many patients lose the device sometimes without becoming aware of it. From the point of view of patient acceptability, a liquid dosage form is preferable.
This study will focus on recent findings on the mechanism of formulation effects in ocular bioavailability, employing a polymer for the preparation of hydro gels, bioadhesive dosage forms and system including liposome and nanoparticles. Bioadhesive systems can be either polymeric solutions or micro particle suspensions. Such polymers have demonstrated premising improvements in the ocular bioavailability by increasing the drug residence time in the precorneal area or diminishing side-effects due to systemic absorption and diminishing the necessary amount of drug for a therapeutic response in the anterior chamber.6
Numerous studies have been conducted on polymer viscolyzers, bioadhesive delivery system and colloidal systems, all in rabbits and in humans. Bioadhesive properties of polymers seemed to be related to precorneal retention of the drug more significantly in comparison with other isoviscous and non-bioadhesive polymers. Encapsulation of drugs in liposomes and nanoparticles was correlated to an increase of the drug concentration in the ocular tissues. There is a need for a polymer in which drug could be trapped physically to prolong drug residence time on the corneal surface and preserve visual acuity. Such system should be probably more hydrophobic than the materials currently employed, and would have to exhibit pseudoplastic behavior to minimize interference with blinking.7-12
Materials and Methods
Materials
Ciprofloxacin hydrochloride was gifted by Kauks Pharma Care, Faridabad, Hariyana (India). Gelrite was procured from Burzin and Leones Pvt. Ltd., Mumbai (India). All other chemicals used were of reagent grade.
Preparation of ocular inserts
Ocular inserts of Ciprofloxacin hydrochloride (0.045 mg/insert) were prepared by surface casting method using mercury as the substrate (Mundada and Shrikhande, 2006). Required amount of gelrite was dissolved in purified water at 800C, with the aid of stirring. To this glycerol (1%, w/w) as plasticizer and calculated amount of Ciprofloxacin hydrochloride were added, and stirred till homogenous. The solution was poured over a glass ring on the mercury surface and covered with an inverted funnel to allow slow and uniform evaporation at room temperature for 48 hr. The films so obtained were punched with the help of a sharp edged die.
Analysis of Ciprofloxacin hydrochloride
UV spectrophotometric method was used for analysis of Ciprofloxacin hydrochloride.
V. Scan
A solution of ciprofloxacin hydrochloride was prepared in Sorenson phosphate buffer (pH 7.4) and scanned for UV absorption. The solution of ciprofloxacin hydrochloride showed maximum UV absorption at 276.5 nm.
Stock solution
100 mg of ciprofloxacin hydrochloride was accurately weighed and dissolved in 100 ml of Sorenson phosphate buffer (pH 7.4) to get solution of 1000 μg/ml. From this solution 1ml was withdrawn and diluted upto 100 ml with Sorenson phosphate buffer (pH 7.4) to get ciprofloxacin hydrochloride stock solution of 10 μg/ml.
Standard solutions
From above stock solutions aliquot of 2, 4, 6, and 8 ml were withdrawn to get concentration range of 2-10 μg/ml.
Preparation of standard Curve
The absorbance of standard prepared above was measured at λmax 276.5 nm for ciprofloxacin hydrochloride. The data so obtained was plotted for concentration in X axis and absorbance in Y axis and regression was carried out.
Figure 1 – Calibration curve of ciprofloxacin hydrochloride in Sorenson phosphate buffer (pH 7.4).
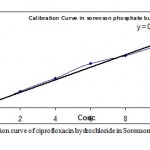 |
Figure 1: Calibration curve of ciprofloxacin hydrochloride in Sorenson phosphate buffer (pH 7.4).
|
The equation of line for calibration curve of ciprofloxacin hydrochloride in Sorenson phosphate buffer (pH 7.4) was found to be y = 0.0133x + 0.0068 (R2 = 0.9872)
Formulation and Evaluation of ocular inserts
Formulation of ocular film of gelrite (Table 1)
| S.No. | Concentration %w/v | 0.25 | 0.50 | 0.75 | 1.00 |
| Formulation code | OFGL1 | OFGL2 | OFGL3 | OFGL4 | |
| 1 | Drug (gm) | 0.045 | 0.045 | 0.045 | 0.045 |
| 2 | Plasticizer (%) | 1 | 1 | 1 | 1 |
| Qty | 100 patches | 100 patches | 100 patches | 100 patches |
Thickness of the film
Thickness of the recovered films was measured using screw gauze. After performing the initial settings the film was placed on the anvil such that area where the thickness is to be measured lies. The screw was gently tighted on to the specimen and reading of the gauze was noted to get the thickness of the film.
Weight variation test: Three films of each formulation were randomly selected and weighed individually then the mean weight of films of each batch was calculated.
Surface pH determination
The surface pH determination of the film was done by allowing them to swell by placing 2 drops of distilled water over it. After this the swollen film was taken and pH was determined using pH paper on the surface of the film.
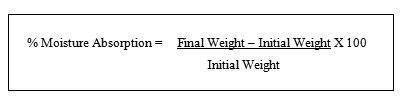
Percentage moisture absorption
The percentage moisture absorption test was carried out to check physical stability or integrity of the ocusert. Ocusert films were weighed and placed in a desiccator containing 100 ml of saturated solution of ammonium chloride (which provide 79% humidity). After 3 days the ocuserts were taken out and reweighed. The percentage moisture absorption was calculated using the formula
Percentage moisture loss
Percentage moisture loss was carried out to check integrity of the film at dry condition. Ocuserts were weighed and kept in the desiccators containing anhydrous CaCl2. After 3 days the ocuserts were taken out and reweighed, the percentage moisture loss was calculated using the formulae

Assay of drug in the film
To check the uniformity of the drug in the cast film, insert were cut at different places in the cast films and each film was dissolved in the 5 ml phosphate buffer, shaken for 6 hrs. The content was filtered out with the help of 0.5 μm syringe filter and 3 ml of the filtrate was taken in a quartz cell and observe the absorbance, calculate to get the amount of ciprofloxacin in each film.
In vitro release study
Cellulose membrane was mounted between clamped donor and receptor cells of modified version of Franz diffusion cell. Cell was placed on magnetic stirrer in holding position. The receptor cell (internal volume 14 ml) was filled with simulated tear fluid (pH 7.4) and stirred using Teflon coated magnetic stir bar. Drug solution (1 ml) was placed on film in donor cell and stirring of the receptor fluid (jacketed with water at 34±1°C) was started. Sealing a glass cover slip over donor cell with silicon grease diminished evaporation of drug solution. Samples (1 ml) were withdrawn from side arm of receptor cell at 15, 30, 45, 60, 120, 180, 240 and 360 minutes, and withdrawn samples were replaced with equal volume of fresh solution. Withdrawn samples were analyzed spectrophotometrically (Shimadzu UV 1700) measuring absorbance at lmax of 276.5 nm.
Results and Discussion
The results of thickness, weight variation, surface pH and drug content uniformity are shown in Table 2. The inserts were found to possess uniform thickness and weight within the batch. It was found that the thickness and weight of inserts were increased with the increasing concentration of gelrite, with batch OFGL4 (1.00 % w/v, gelrite) having thickness of 26.7 μm and weight of 1.42 mg, The drug content was consistent in all batches and varied from 100.2 % to 99.0%. As the surface pH of all the inserts was found to be approx. 7.2, they were not expected to cause irritation.
Table 2: Physiochemical characteristics of various batches of Gelrite based ocular inserts
| BatchCode | Gelrite Conc.(%,w/v) | Weight (mg) | Thickness (μm) | Surface pH |
Drug Content (%)
|
In vitro release |
| OFGL1 | 0.25% | 0.45 | 2.1 | 7.3 | 100.2 | 98.50 |
| OFGL2 | 0.5% | 0.83 | 5.9 | 7.2 | 98.6 | 97.59 |
| OFGL3 | 0.75% | 1.19 | 12.6 | 7.2 | 98.9 | 92.62 |
| OFGL4 | 1.00% | 1.42 | 26.7 | 7.2 | 99.0 | 86.52 |
Conclusion
In the present study, an attempt was made to enhance the precorneal residence time of drugs using different polymer based ocular insert which are most commonly employed for antibacterial action were used as the model drug.
Ocular inserts OFGL4 (1% gelrite) containing 0.045 mg ciprofloxacin hydrochloride was casted. The inserts were evaluated for mechanical strength, thickness, weight variation, surface pH, assay of drug and in vitro release effect. The inserts was found to be of uniform thickness and weight. Assay of the inserts revealed uniformity of the drug contents within the batch of inserts.
Acknowledgement
The authors are grateful to Kauks Pharma Care, Faridabad, Hariyana (India) for providing the gift samples of Ciprofloxacin hydrochloride and Department of Pharmaceutical Sciences, Raj Kumar Goel Institute of Technology, Ghaziabad (U.P.), India for providing the necessary research facilities.
References
- Venkateshwar, R. Somashekar,S. Preparation and Evaluation of Ocular inserts containing Norfloxacin . Turk. J. Med. Sci. 2004; 34 : 239-246.
- Sreeraj M, Ashim K. M. overview of ocular drug delivery. Marcel dekker.
- Aqil, Dr. Mohd. Advances in Ophthalmic Drug Delivery System: Part I &II. 12th April 2005 (http://www.Pharmainfo. Net).
- Merkli A., Tabatabay C., Gurny R.:Use of Insoluble Biodegradable Polymrs in Ophtalmc Systems for the Sustained Release of Drugs Eur.J.Pharm. Biopharm.: 41 (5); 271-283; ( 1995 ).
- Le Bourlais A., Treupel-Acar L.; Rhodes C.T.; Sado P.A.; Leverge R. New Ophtalmic Drug Delivery System Drug Develop. Ind. Pharm.: 21 ( I ): 19-59; ( 1995.
- Greaves J.L.; Olejnik 0.; Wilson C.G. Polymers and the Precorneal Tear Film S.T.P. Pharma Sciences: 2 ( 1); 13-33; ( 1992).
- Schoenwald R.D.: Ocular Drug Delivery: Pharmacokinetic Considerations clinical Pharmacokinetics: 18 ( 4 ); 255- 269; 1990.
- Lee V.H.: Mechanisnis and Facilitation of Corneal Drug Penetration Journal of Controll. Releas.: 11; 79-90; ( 1990 ).
- Keister J.C.; Cooper E.R.; Missel P.J.; Lang J.C.; Hager D.F. Limts on Optimizing Ocular Drug Delivery J. Pharm. Sci.: 80 (1 ); 50-53; ( 1991 ).
- Robinson J.R.: Ocular Drug Delivery. Mechanism(s) of Corneal Drug Transport and Muchadhesive Delivery Systems S.T.P. Pharma: 5 ( 12 ); 839-846; ( 1989).
- Peyman G.A.; Ganiban G.J.: Delivery Systems for Intraocular Routes, Advanced Drug Delivery Reviews: 16; 107- 123; ( 1995 ).
- Jarvinen K., Jarvinen T. and Urtti A.: Ocular absorption following topical delivery: Advanced Drug Delivery Reviews: 16; 3-19; ( 1995 )
- Vyas S P, Khar R K, Controlled Drug Delivery, Concepts and Advances. Vallabh Prakashan, pp. 382-407.
- Kumar S, Haglund B O, Himmelstein K J. In-situ forming gels for ophthalmic drug delivery. J. Ocular Pharmacol. 1994;10 (1): 47-56.
- Hendrickson, A.E. and Youdelis, C. (1984). The morphological development of the human fovea. Ophthalmology 91, 603-612.
- Mann, (1964). The development of the human eye. Grune and Stralton, New York.
- Ogden, T.E. (1989) Retina: Basic Science and inherited retinal disease, vol 1. The CV Mosby Co. St. Louis.
- Indian Pharmacopoeia 1996, Vol-I, 189-190.

This work is licensed under a Creative Commons Attribution 4.0 International License.





